長庚生物標識中心
長庚生物標識中心
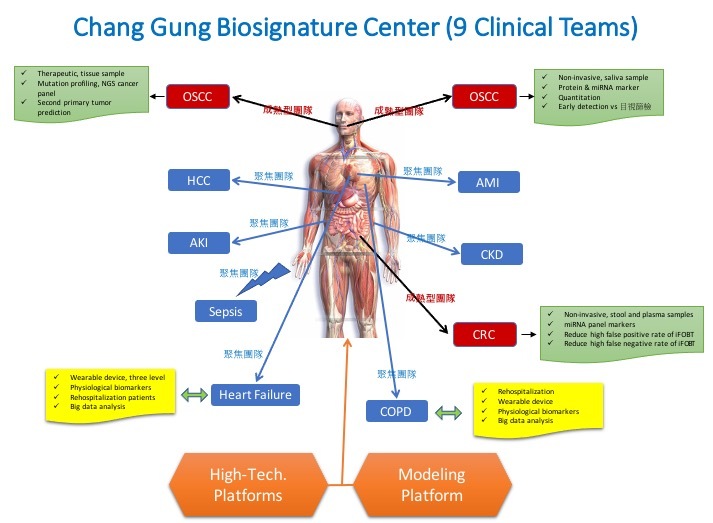
長庚生物標識中心 (Chang Gung Biosignature Center, CGBC) 針對國人常見之九種疾病進行深入研究探討,包括:口腔癌、大腸直腸癌、肝癌、急性腎衰竭、慢性腎損傷、急性冠心病、敗血症、心臟衰竭及慢性肺阻塞疾病等研究計畫。經過多年的深入研究,目前已有數個計畫即將進入臨床試驗階段,並有數個研究成果與技術進入專利申請與技轉合作階段。例如:口腔癌早期診斷生物標識計畫 (此計畫是配合國家對口腔癌篩檢之政策與國健署共同合作推動)、口腔癌預後指標生物標識計畫、大腸直腸癌生物標識計畫 (此計畫是配合國家對大腸直腸癌篩檢之政策與國健署共同合作推動)、肝癌生物標識計畫及國人常見之急、慢性腎臟疾病等計畫,皆有重要且豐碩的成果。在生物標識研究上,長庚體系是全台最早進入臨床試驗的團隊,並在諾貝爾獎得主Prof. Leland Hartwell、黃秉乾院士及核心委員的規劃下,必將成為此領域的世界頂尖一流團隊。
透過長庚生物標識研究建立『技術及生物資訊平台』,支援並積極參與九項重大疾病的生物標識研究計畫,成功建立並使用了多種高通量體學分析技術及生物資訊分析平台,包括蛋白質體、代謝體、次世代定序、基因體RT-qPCR、生物資訊分析以及人工智慧分析平台,全力投入轉譯應用研究,且已有相當亮眼的成果。成功建置此一完善生物標識研究環境(軟硬體、多體學分析技術與專業團隊),使長庚體系生物標識研究能在國際上凸顯特色,並將成果轉譯於臨床。
重要成果
(1)針對臨床團隊需求,開發高通量檢體處理技術並優化實驗流程,以涵蓋不同類型檢體(細胞株、組織、糞便及各種體液樣本如血液、唾液、尿液等);
(2)協助臨床團隊完成大量實驗檢體(每年約完成5,000個)分析及實驗數據處理;
(3)支援整合型生物標識計畫發表數十篇國際論文,申請14個生物標記相關研究發明專利,已有多個專利獲證及一組標記技轉;
(4)建置完善生物標識研究環境所需軟硬體,同時培育包含數十位年輕教師、醫師、研究員、博士後、助理及博碩士學生的長庚生物標識研究專業團隊。
PNAS October 11, 2016 113 (41) 11549-11554.
Saliva protein biomarkers to detect oral squamous cell carcinoma in a high-risk population in Taiwan
PMID: 27663741 PMCID: PMC5068314 DOI: 10.1073/pnas.1612368113
Jau-Song Yu 1, Yi-Ting Chen 2, Wei-Fan Chiang 3, Yung-Chin Hsiao 4, Lichieh Julie Chu 4, Lai-Chu See 5, Chi-Sheng Wu 6, Hui-Tzu Tu 7, Hsiao-Wei Chen 6, Chia-Chun Chen 8, Wei-Chao Liao 6, Ya-Ting Chang 6, Chih-Ching Wu 9, Che-Yi Lin 10, Shyun-Yeu Liu 10, Shu-Ti Chiou 11, Shu-Li Chia 12, Kai-Ping Chang 13, Chih-Yen Chien 14, Su-Wei Chang 15, Chee-Jen Chang 16, John D Young 17, Chia C Pao 18, Yu-Sun Chang 19, Leland H Hartwell 20
Abstract
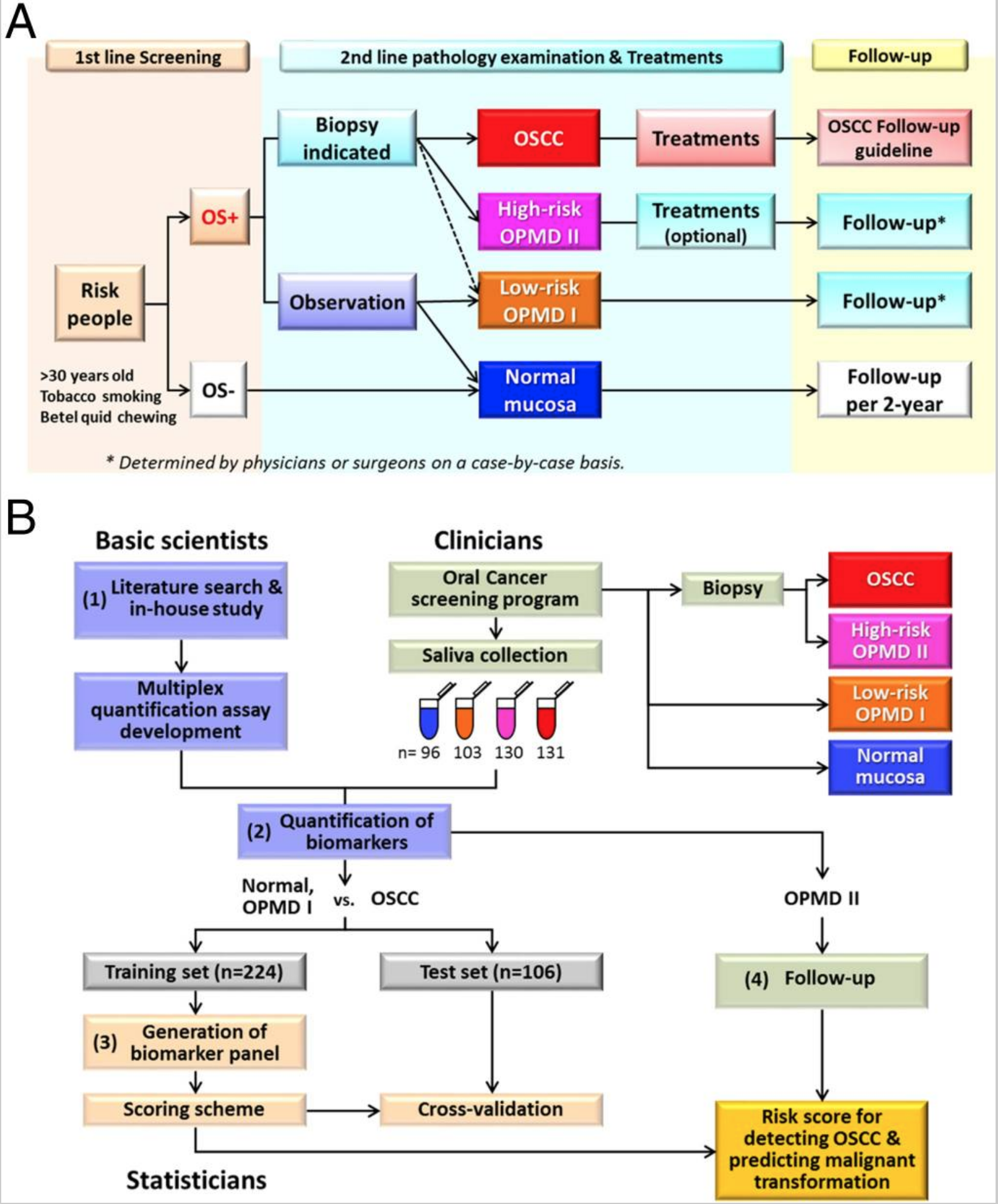
Most cases of oral squamous cell carcinoma (OSCC) develop from visible oral potentially malignant disorders (OPMDs). The latter exhibit heterogeneous subtypes with different transformation potentials, complicating the early detection of OSCC during routine visual oral cancer screenings. To develop clinically applicable biomarkers, we collected saliva samples from 96 healthy controls, 103 low-risk OPMDs, 130 high-risk OPMDs, and 131 OSCC subjects. These individuals were enrolled in Taiwan's Oral Cancer Screening Program. We identified 302 protein biomarkers reported in the literature and/or through in-house studies and prioritized 49 proteins for quantification in the saliva samples using multiple reaction monitoring-MS. Twenty-eight proteins were successfully quantified with high confidence. The quantification data from non-OSCC subjects (healthy controls + low-risk OPMDs) and OSCC subjects in the training set were subjected to classification and regression tree analyses, through which we generated a four-protein panel consisting of MMP1, KNG1, ANXA2, and HSPA5. A risk-score scheme was established, and the panel showed high sensitivity (87.5%) and specificity (80.5%) in the test set to distinguish OSCC samples from non-OSCC samples. The risk score >0.4 detected 84% (42/50) of the stage I OSCCs and a significant portion (42%) of the high-risk OPMDs. Moreover, among 88 high-risk OPMD patients with available follow-up results, 18 developed OSCC within 5 y; of them, 77.8% (14/18) had risk scores >0.4. Our four-protein panel may therefore offer a clinically effective tool for detecting OSCC and monitoring high-risk OPMDs through a readily available biofluid.
Keywords: biomarkers; early detection; oral cancer.